Heart Failure with Reduced Ejection Fraction
Heart failure is a common complex clinical syndrome of symptoms and signs caused by impairment of the heart’s action as a pump supporting the circulation.
Heart Failure with Reduced Ejection Fraction (HR-REF), previously known as “systolic” heart failure, is characterised by a reduced left ventricular ejection fraction.
There is no single diagnostic test. Use clinical judgement based on
history, physical examination and investigations.
Diagnosis may be confirmed by a combination of:
- symptoms or signs of heart failure
- raised serum NT-proBNP >400ng/l
- echocardiography
- specialist assessment (recommended)
Reference
1)National Institute for Health and Care Excellence. Chronic Heart Failure in Adults: diagnosis and management [Internet]. [London]: NICE; 2018 (NICE guideline [NG181])
Heart failure may be classified by symptoms and/or left ventricular ejection fraction (LVEF):
New York Heart Association (NYHA) Functional Classification1
| Class | Description | |
| I | No limitation of physical activity | Ordinary physical activity does not cause undue fatigue, palpitation, shortness of breath |
| II | Slight limitation of physical activity | Ordinary physical activity results in fatigue, palpitation, shortness of breath |
| III | Marked limitation of physical activity | Comfortable at rest. Less than ordinary activity causes fatigue, palpitation, or shortness of breath |
| IV | Unable to carry on any physical activity without discomfort | Symptoms of heart failure at rest. If any physical activity is undertaken, discomfort increases |
Categories of left ventricular ejection fraction
- Definitions of normal LVEF and degrees of its impairment are varied.
- Historically, a LVEF of >50% has been regarded as normal, and a LVEF of <40% as abnormal.
- Certain treatment options are recommended only to patients with particular levels of reduced LVEF.
This figure gives an overview of current categorisations2.
- If it feels confusing, it’s because it is. The main thing to appreciate is the varing definition of normal between 40% and 50%. In practice, we will probably be led by local expert opinion on a case-by-case basis.
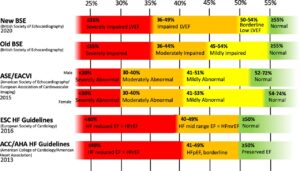 |
|
Reproduced with permission from Hudson et al.2 |
References
1)Dolgin M, Association NYH, Fox AC, Gorlin R, Levin RI, New York Heart Association. Criteria Committee. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Boston, MA: Lippincott Williams and Wilkins; March 1, 1994
2)What is ‘normal’ left ventricular ejection fraction?
Untreated, heart failure is progressive and carries a poor prognosis.
- Before modern treatments, 5-year survival rates were 30-40%1.
Prognosis is complex, and depends on age, setting, sex and other factors.
These selected figures are a guide, though there is significant uncertainty:
- survival rates in the context of standard care at the time
- they combine data on both HF-REF (reduced) and HF-PEF (preserved) ejection fraction
Overall survival rates
| Survival rates at: | 1 year | 5 years | 10 years | 15 years |
| 81% | 48% | 26% | 13% | |
|
Year of initial diagnosis* |
2016 |
2012 |
2007 |
2000 |
* Defined by first recorded clinical code
Source: UK Primary Care database study 20192
Survival rates by age at diagnosis
more
- HF-PEF and HF-REF combined
| Survival rate at: | 1 year | 5 years | 10 years | 15 years |
| Age: 45-54 years | 90% | 79% | 65% | 54% |
| 55-64 | 88% | 71% | 52% | 38% |
| 65-74 | 84% | 59% | 35% | 17% |
| 75-84 | 77% | 43% | 18% | 6% |
| 85-94 | 63% | 22% | 4% | 0.2% |
| ≥ 95 | 44% | 6% | – | – |
Source: UK Primary Care database study 20192
Survival rates for HF-REF v HF-PEF v LVSD without HF
more
- HF-REF carries a worse prognosis than HF-PEF
| Survival rate at: | 5 years | 10 years |
| HF-REF | 53% | 27% |
| HF-PEF | 62% | 26% |
| LVSD* ≤40% but no HF | 69% | 38% |
* LVSD, left ventricular systolic dysfunction
Source: UK Primary Care cohort study 20143
- patients identified from UK primary care lists and pro-actively screened for heart failure and asymptomatic LVSD
- diagnosed between 1995 and 1999, followed up until 2009
Similar differences are seen in studies around the world over time:
| Survival rate at: | 5 years |
| HF-REF | 63% |
| HF-PEF | 70% |
Source: Systematic review 20191 (pooled data from studies 1987-2017)
Survival rates by NT-proBNP level
more
- HF-PEF and HF-REF combined
| Survival rate at: | 1 year | 5 years | 10 years |
| NT-proBNP*: <400 | 79% | 54% | 30% |
| 400-1999 | 81% | 50% | 24% |
| ≥2000 | 73% | 38% | 18% |
*NT-proBNP measured at time of diagnosis, pg/ml.
Source: UK Primary Care database study 20204
Survival rates by diagnosis at hospital admission v in community
more
- HF-PEF and HF-REF combined
Survival rates are worse for those first diagnosed during acute hospital admission
| Survival rate at: | 1 year | 5 years | 10 years | 15 years |
| Diagnosed in community | 81% | 52% | 29% | 15% |
| Diagnosed at hospital admission | 69% | 37% | 18% | 8% |
Source: UK Primary Care database study 20192
Survival rates men v women
more
- Age adjusted mortality rates are roughly the same for men and women.
- However, women are diagnosed on average 5 years older than men, so have higher crude mortality rates5.
References
more
1)Jones NR, Roalfe AK, Adoki I et al. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail 2019; 21: 1306-1325
2) Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: population based cohort study
3), , Iles R, Hobbs FR. Ten-year prognosis of heart failure in the community: follow-up data from the Echocardiographic Heart of England Screening (ECHOES) study. Eur J Heart Fail 2012; 14: 176-184
4), et al. Natriuretic peptide level at heart failure diagnosis and risk of hospitalisation and death in England 2004–2018
5)Taylor CJ, Ordóñez-Mena JM, Jones NR et al. National trends in heart failure mortality in men and women, United Kingdom, 2000–2017. Eur J Heart Fail 2021, 23: 3-12
There are many treatment options available which improve symptoms and prognosis.
- Most people would benefit from a multiple, additive treatment strategy – this is reflected in NICE guidance.
However, many patients are older, frailer, with more co-morbidities than those who participated in the clinical trials.
- Additive treatments can cause interactions and side effects.
- Personalised care, close monitoring and multi-disciplinary input are key in maximising benefit and minimising harm in this complex condition.
Treating HF-REF with CKD
CKD is a common comorbidity with HF-REF. It may increase the risk of treatment harms such as hyperkalaemia and deteriorating renal function.
The NICE guideline from 2018 reviewed evidence for HF treatments in populations with CKD. It found:
- the benefits of the three main treatments in the guideline (ACEi, BB and MRA) would apply to patients with CKD
- VERY LOW quality evidence, mainly based on subgroup analysis of trial data, means there is significant uncertainty
The NICE recommendations, based on the limited evidence and clinical experience, are:
- with CKD with eGFR >30 ml/min/1.73m2, offer ACEi, BB and MRAs as per the standard guideline recommendations
- if eGFR <45 ml/min/1.73m2, consider lower doses and slower titration
- if eGFR <30 ml/min/1.73m2, liaise with specialist MDT and renal physician
- monitor the response to titration of medicines closely, taking into account the increased risk of hyperkalaemia
Treatment options:
Diuretics reduce fluid overload and heart failure symptoms.
They have no proven prognostic benefit other than managing acute or chronic fluid overload.
- Patients may not need them indefinitely once stabilised on other treatment.
- This may be a good opportunity to de-prescribe especially if polyuria is impacting lifestyle and social opportunities.
- If deprescribing, be alert for recurrence of fluid retention.
Side effects are dose-dependent and include:
- electrolyte disturbance (hypokalaemia, hyponatraemia)
- hypotension, dizziness
- acute kidney injury
Source: BNF
ACE inhibitors (and ARBs) reduce mortality and hospitalisation in HF-REF.
The greater the degree of left ventricular impairment, the greater the benefit.
- Benefits begin to accrue within 6 weeks.
If 100 people with LVEF <23% are treated with an ACE inhibitor for 4 years, 8.3 will avoid death compared with if they hadn't taken an ACE inhibitor
If 100 people with LVEF <23% are treated with an ACE inhibitor for 4 years, 10 will avoid a hospital admission compared with if they hadn't taken an ACE inhibitor
If 100 people with LVEF <23% are treated with an ACE inhibitor for 4 years,13.4 will avoid death or hospital admission or MI compared with if they hadn't taken an ACE inhibitor
If 100 people with LVEF 23-27% are treated with an ACE inhibitor for 4 years, 4 will avoid death compared with if they hadn't taken an ACE inhibitor
If 100 people with LVEF 23-27% are treated with an ACE inhibitor for 4 years, 7 will avoid a hospital admission compared with if they hadn't taken an ACE inhibitor
If 100 people with LVEF 23-27% are treated with an ACE inhibitor for 4 years, 8 will avoid death or hospital admission for HF or MI compared with if they hadn't taken an ACE inhibitor
If 100 people with LVEF 28-35% are treated with an ACE inhibitor for 4 years, 2 will avoid death compared with if they hadn't taken an ACE inhibitor
If 100 people with LVEF 28-35% are treated with an ACE inhibitor for 4 years, 5 will avoid hospital admission for HF compared with if they hadn't taken an ACE inhibitor
If 100 people with LVEF 28-35% are treated with an ACE inhibitor for 4 years, 5 will avoid death or hospital admission for HF or MI compared with if they hadn't taken an ACE inhibitor
If 100 people with LVEF >35% are treated with an ACE inhibitor for 4 years, 2 will avoid death compared with if they hadn't taken an ACE inhibitor
If 100 people with LVEF >35% are treated with an ACE inhibitor for 4 years, 2 will avoid hospital admission for HF compared with if they hadn't taken an ACE inhibitor
If 100 people with LVEF 28-35% are treated with an ACE inhibitor for 4 years, 5 will avoid death or hospital admission for HF or MI compared with if they hadn't taken an ACE inhibitor
Evidence Source: NICE
Figures derived from a systematic review1 included in the 2003 NICE guideline2 (and are current for the 2018 guideline).
- 5 randomised controlled trials
- included 12,763 patients
- compared ACE inhibitor to placebo
- followed up for 1-4 years
References
1)Flather M, Yusuf S, Køber L et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. Lancet 2000; 355(9215): 1575-1581
2)National Collaborating Centre for Chronic Conditions (UK). Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK); 2003. (NICE Clinical Guidelines, No. 5.) 7, Treating heart failure
Evidence Quality: HIGH
| This research provides a very good indication of the treatment effect.
However, it is possible that the true effect is slightly smaller or greater. |
NICE rated this evidence as HIGH quality1:
- randomised controlled trials at low risk of bias
- individual patient data meta-analysis
- statistically significant findings with reasonable confidence intervals
- relevant to a typical population
Data detail
Results for total mortality in groups with LVEF 28-35% and >35% did not reach statistical significance.
Result for hospital admissions for HF in the group with LVEF >35% did not reach statistical significance.
However, it is likely that these data provide a reasonable estimate of treatment effect.
Reference
1)National Collaborating Centre for Chronic Conditions (UK). Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK); 2003. (NICE Clinical Guidelines, No. 5.) 7, Treating heart failure
Study Population
The population characteristics in this review1 were:
- mean age 61
- 19% female
- ethnicity only reported for about half of the population: 80% Caucasian, 15% Black.
- 54% previous MI
- mean LVEF 29%
- mean BP 122/76mmHg
- 18% diabetes
- 35% hypertension
- 36% smokers
- 21% beta-blockers
Reference
1)Flather M, Yusuf S, Køber L et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. Lancet 2000; 355(9215): 1575-1581
ARBs (angiotensin receptor blockers)
ARBs are recommended as an alternative for people unable to tolerate ACE inhibitors (usually due to cough).
Evidence shows they reduce hospitalisation and mortality in patients with HF-REF, but:
- evidence is not as strong as that for ACE inhibitors
- trials were undertaken in recent years when patients had a better prognosis due to concomitant treatments such as beta-blockers1
| Placebo | ARB | Absolute Risk Reduction | Number Needed to Treat | |
| HF hospitalisation | 18.5% | 12% | 5.5% | 18 |
| HF hosp + CV death | 40% | 33% | 7% | 14 |
| Absolute Risk Increase | Number Needed to Harm | |||
| Hyperkalaemia | 0.3% | 1.9% | 1.6% | 63 |
| Hypotension | 1.3% | 3.9% | 2.6% | 38 |
- MODERATE quality evidence. Figures after approximately 2 years of follow-up
| This research provides a good indication of the treatment effect.
There is a moderate possibility that the true effect is smaller or greater. |
Reference
1)National Clinical Guideline Centre (UK). Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care: Partial Update [Internet]. London: Royal College of Physicians (UK); 2010 Aug. (NICE Clinical Guidelines, No. 108)
Side-effects of ACE inhibitors in the clinical trials of patients with HF-REF from a systematic review1:
| Placebo | ACE inhibitor | Absolute Risk Increase | Number Needed to Harm | |
| Cough | 22% | 28% | 6% | 17 |
| Hypotension | 11% | 16% | 5% | 20 |
| Dizziness | 27% | 31% | 4% | 23 |
| Hyperkalaemia | 3% | 6% | 3% | 33 |
| Increase in creatinine | 6% | 8% | 2% | 50 |
- MODERATE Quality Evidence
| This research provides a good indication of the treatment effect.
There is a moderate possibility that the true effect is smaller or greater. |
Angioedema is estimated to occur in between 1 in 10 – 100 patients
- highly variable incidence reported in research studies
- more common in Black patients
Source: BNF
Reference
1)Bœuf-Gibot S, Pereira B, Imbert J et al. Benefits and adverse effects of ACE inhibitors in patients with heart failure with reduced ejection fraction: a systematic review and meta-analysis. Eur J Clin Pharmacol 2021; 77: 321–329
Beta-blockers (licensed for heart failure) reduce hospitalisations and mortality in patients with HF-REF.
Benefits are similar across NYHA Classes II-IV, though data is limited.
- Risk reductions begin to be seen after one year of treatment.
If 100 people with NHYA class II-III are treated with a beta-blocker for 1 year, 4 will avoid death compared with if they hadn't taken a beta-blocker
If 100 people with NHYA class II-III are treated with a beta-blocker for 1 year, 1 will avoid death from worsening heart failure compared with if they hadn't taken a beta-blocker
If 100 people with NHYA class II-III are treated with a beta-blocker for 1 year, 3 will avoid sudden death compared with if they hadn't taken a beta-blocker
If 100 people with NHYA class II-III are treated with a beta-blocker for 1 year, 5 will avoid a hospital admission for heart failure compared with if they hadn't taken a beta-blocker
If 100 people with NHYA class II-III are treated with a beta-blocker for 1 year, 6.6 will avoid death or hospital admission for HF compared with if they hadn't taken a beta-blocker
Evidence Source: NICE
NICE guideline review 20031 references a 2001 systematic review2 (and is current for the 2018 guideline):
- 22 randomised controlled trials included 10,480 patients
- compared beta-blockers to placebo
- follow up average 1 year
The data presented in the graphics above is taken from a large key trial3 within this review. It is reasonably representative of a primary care population (see Study Population).
References
1)National Collaborating Centre for Chronic Conditions (UK). Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK); 2003. (NICE Clinical Guidelines, No. 5.) 7, Treating heart failure
2)Shibata MC, Flather MD, Wang D. Systematic review of the impact of beta blockers on mortality and hospital admissions in heart failure. Eur J Heart Fail 2001; 3(3): 351-7
3)Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF).The Lancet 1999; 353(9169): 2001-2007
Evidence Quality: HIGH
| This research provides a very good indication of the treatment effect.
However, it is possible that the true effect is slightly smaller or greater. |
NICE rated this evidence as HIGH quality1
- large populations in randomised controlled trials
- low risk of bias
- relevant to the target population
- consistent effects demonstrated with reasonable confidence intervals
References
1)National Collaborating Centre for Chronic Conditions (UK). Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK); 2003. (NICE Clinical Guidelines, No. 5.) 7, Treating heart failure
Study Population
The population characteristics in the trial of 4000 people1 providing this data were:
- mean age 64
- 23% female
- ethnicity: 94% White, 5% Black, 1% other
- co-existing conditions:
- 65% ischaemic heart disease (48% previous MI)
- 44% hypertension
- 25% diabetes
- 16% atrial fibrillation
- 96% ACEi/ARB therapy
- mean BP 130/80mmHg
- mean baseline heart rate 83bpm
Reference
1)Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF). Lancet 1999; 353(9169): 2001-2007
Which beta-blocker?
Beta-blockers with a licence for the treatment of heart failure in the UK are:
- bisoprolol
- carvedilol
- nebivolol
Source: BNF
Beta-blockers and co-morbidities
Beta-blockers are safe to use for HF-REF with these co-morbidities1:
- COPD
- no adverse effect on FEV1 or respiratory symptoms2
- peripheral Vascular Disease
- no adverse effect on walking capacity or symptoms of intermittent claudication in patients with mild to moderate peripheral arterial disease3
- diabetes
- may cause a small increase plasma glucose levels, though benefits from treating HF-REF are likely to outweigh any potential harms
- a 2015 systematic review found limited LOW quality evidence from small, short term trials4 showing:
- 1.33 mmol/L increase in fasting plasma glucose with non-selective beta-blockers (including carvedilol)
- no increase in fasting plasma glucose with cardioselective beta-blockers (atenolol and nebivolol). Bisoprolol is cardioselective but was not studied in this review.
Caution is needed in asthma (or reversible airways disease) as beta-blockers can induce bronchospasm.
- However, cardioselective beta-blockers may well be safe for most.
- Initiate cardioselective beta-blocker under specialist supervision for those with asthma and HF-REF (Source: BNF)
- A 2002 Cochrane review5 of small, short term randomised trials of cardio-selective beta-blockers in patients with stable, mild-moderate, reversible airways disease showed no adverse respiratory effects.
- A 2021 review6 of observational data and global safety reports found no evidence of asthma exacerbations or death associated with cardioselective beta-blockers.
References
1)Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care: Partial Update [Internet]. London: Royal College of Physicians (UK); 2010 Aug. (NICE Clinical Guidelines, No. 108.) 4, Diagnosing heart failure
2)Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No.: CD003566
3)Radack K, Deck C. Beta-adrenergic blocker therapy does not worsen intermittent claudication in subjects with peripheral arterial disease. A meta-analysis of randomized controlled trials. Arch Intern Med 1991 Sep; 151(9): 1769-76
4)Hirst JA, Farmer AJ, Feakins BG et al. Quantifying the effects of diuretics and β-adrenoceptor blockers on glycaemic control in diabetes mellitus – a systematic review and meta-analysis. British Journal of Clinical Pharmacology 2015; 79(5): 733–743
5)Salpeter SR, Ormiston TM, Salpeter EE, Wood‐Baker R. Cardioselective beta‐blockers for reversible airway disease. Cochrane Database of Systematic Reviews 2002, Issue 4. Art. No.: CD002992
6). The safety of cardioselective β1-blockers in asthma: literature review and search of global pharmacovigilance safety reports.
Rates of side effects of beta-blockers reported in the key trials on HF-REF1
| Placebo | Beta-blocker | Absolute Risk Increase | Numbers Needed to Harm per year* | |
| Hypotension | 6.1% | 7.6% | 1.5% | 91 |
| Dizziness | 16.6% | 21.5% | 4.9% | 17 |
| Bradycardia | 1.8% | 5.7% | 3.9% | 26 |
| Fatigue | 22.4% | 23.6% | 1.2% | 297 |
|
* The standardised calculation for Number Needed To Harm per year was calculated by the review authors. The other figures are pooled from trials ranging from 6-24 months duration. |
||||
LOW quality evidence
- wide confidence intervals and not all differences reached statistical significance
- patients in clinical trials are younger and with fewer co-morbidities on average than those in clinical practice
| This research provides some indication of the treatment effect.
There is a high possibility that the true effect is smaller or greater. |
Reference
1) Ko DT, Hebert PR, Coffey CS et al. Adverse Effects of β-Blocker Therapy for Patients With Heart Failure: A Quantitative Overview of Randomized Trials. Arch Intern Med 2004; 164(13): 1389–1394
For patients with HF-REF who continue to have symptoms of heart failure despite treatment with both an ACEi/ARB and a beta-blocker, NICE recommends offering a mineralocorticoid receptor antagonist (MRA).
MRAs can improve symptoms, and reduce mortality and hospitalisations in this population.
They are thought to operate via a variety of mechanisms, not just their diuretic effect.
The balance of benefits and harms may be uncertain for individuals.
- Close monitoring is key to the safe use of these drugs (see below).
If 100 people like this are treated with an MRA for 21 months, 3 will avoid death compared with if they hadn't taken an MRA
If 100 people like this are treated with an MRA for 21 months, 7 will avoid a hospital admission for cardiovascular cause compared with if they hadn't taken an MRA
If 100 people like this are treated with an MRA for 21 months, 7.5 will avoid death or hospitalisation for any reason compared with if they hadn't taken an MRA
If 100 people like this are treated with an MRA for 2 years, 8.5 will avoid death compared with if they hadn't taken an MRA
If 100 people like this are treated with an MRA for 2 years, 8 will avoid hospitalisation for a cardiac cause compared with if they hadn't taken an MRA
If 100 people like this are treated with an MRA for 2 years, 10 will avoid a deterioration in their NYHA symptom class compared with if they hadn't taken an MRA
If 100 people like this are treated with an MRA for 2 years, 8 will improve their NYHA symptom class compared with if they hadn't taken an MRA
Evidence Source: NICE
Two trials underpinned the 2018 NICE guideline recommendations1.
They are presented here separately as the populations were different:
- NYHA II, LVEF 26%, on ACE+BB.
-
EMPHASIS HF trial, 2011. 1663 patients. Eplerenone2.
-
- NYHA III-IV, LVEF 25%, on ACE but not BB.
-
RALES trial, 1999. 2737 patients. Spironolactone3.
-
See the “Study Population” section for more details.
References
1)National Institute for Health and Care Excellence. Chronic heart failure in adults: diagnosis and management 2018 [Internet]. [London]: NICE; 2018. (NICE guideline [NG106])
2)Zannad F, McMurray JJV, Krum H et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21
3)Pitt B, Zannad F, Remme WJ et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717
Evidence Quality: LOW
| This research provides some indication of the treatment effect.
There is a high possibility that the true effect is smaller or greater. |
NICE rated this evidence as mostly LOW quality1.
Although data was derived from:
- large randomised controlled trials
- overall low risk of bias
- main outcome measures were statistically significant,
there is uncertainty about the results:
- questionable applicability of the trial populations to all patients with HF-REF
- younger than average, fewer co-morbidities
- in the RALES trial, 90% were not on beta-blockers
- most patients in the trials have more severely reduced LVEF
- some wide confidence intervals
- bias in trial design may have reduced the impression of treatment harms
Reference
1)National Institute for Health and Care Excellence. Chronic heart failure in adults: diagnosis and management 2018 [Internet]. [London]: NICE; 2018. (NICE guideline [NG106])
Study Population
The 2 trials looked at populations with different levels of symptom severity.
Both populations had, on average, severely impaired left ventricular function.
| NHYA Stage II, mean LVEF 26% | NYHA III-IV, mean LVEF 25% |
| EMPHASIS HF trial1 | RALES trial2 |
|
|
Sub-group analyses in both trials suggested a treatment benefit across the spectrum of LV impairment, but neither trial had many patients with moderately or mildly reduced LVEF:
- The guideline recommendation to treat patients based on symptomatology rather than a particular LVEF cut-off is an expert/committee decision based on an assumption of benefit across all classes of LV impairment3.
- Absolute benefits of treatment (ARR and NNT) are likely to be reduced in patients with mild-moderate LV impairment.
References
1)Zannad F, McMurray JJV, Krum H et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21
2)Pitt B, Zannad F, Remme WJ et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709–717
3)National Institute for Health and Care Excellence. Chronic heart failure in adults: diagnosis and management 2018 [Internet]. [London]: NICE; 2018. (NICE guideline [NG106])
Drug dosing and monitoring in the trials
Both trials monitored renal function and potassium, with doses adjusted according to clinical response and/or development of hyperkalaemia or renal dysfunction.
| RALES trial: Spironolactone | EMPHASIS HF trial: Eplerenone |
|
|
Monitoring recommendations
BNF recommendations for monitoring of spironolactone:
Monitor electrolytes—discontinue if hyperkalaemia occurs.
In severe heart failure monitor potassium and creatinine:
- 1 week after initiation and after any dose increase,
- monthly for first 3 months,
- then every 3 months for 1 year, and then every 6 months.
1) Data from RCTs
Harms reported in the 2 key trials are summarised below1,2:
- These are likely to underestimate risk of harms for many patients in practice due to the applicability of the trial populations studied and risk of bias in trial design3.
Risks of hyperkalaemia and renal impairment with MRAs are dose dependent. See below for information on dosing regimens in the trials.
| Placebo | MRA | Absolute Risk Increase | NNH over 2 years | |
| Hyperkalaemia (eplerenone) | 3.7% | 8.0% | 4.3% | 23 |
| Hyperkalaemia (spironolactone) | 5.6% | 19.0% | 13.4% | 7 |
| Mean reduction eGFR (eplerenone) | -1.29 ml/min/1.73m2 | -3.18 ml/min/1.73m2 | – 1.89 ml/min/1.73m2 (mean difference) | n/a |
| eGFR reduction >30% (spironolactone) | 7% | 17% | 10% | 10 |
| Gynaecomastia (eplerenone) | No significant difference | |||
| Gynaecomastia (spironolactone) | 1.3% | 9.1% | 7.8% | 13 |
| Hypotension (eplerenone) | No significant difference | |||
| Hypotension (spironolactone) | Not reported | |||
- LOW-MODERATE quality evidence3
Dosing regimens in RCTs
More
Both trials monitored renal function and potassium, with doses adjusted according to clinical response and/or development of hyperkalaemia or renal dysfunction:
| RALES Trial: Spironolactone | EMPHASIS HF: Eplerenone |
|
|
2) Observational data
A database study from Canada in 20044 showed an increase in hospitalisations and deaths associated with hyperkalaemia in the 3 years following the publication of the RALES trial (which had led to an increase in spironolactone prescribing):
- 50 additional admissions for hyperkalemia for every 1000 new prescriptions for spironolactone issued.
- The authors speculated that this may have been due to poor monitoring, use of higher drug doses or use in patients with other risk factors for hyperkalaemia.
- Though we cannot draw firm conclusions or accurate figures from such a study, it highlights the need for careful thought and close monitoring when using MRAs, especially in more vulnerable patients for whom the metabolic effect of MRAs may be different from that in the patients in the clinical trials.
References
1)Zannad F, McMurray JJV, Krum H et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21
2)Pitt B, Zannad F, Remme WJ et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709–717
3)National Institute for Health and Care Excellence. Chronic heart failure in adults: diagnosis and management 2018 [Internet]. [London]: NICE; 2018. (NICE guideline [NG106])
4)Juurlink DN, Mamdani MM, Lee DS et al. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med 2004; 351: 543–551
Specialist-initiated treatment
NICE recommends dapagliflozin as an option for treating symptomatic HF-REF, but only as an add-on to optimised standard care with:
- ACEi/ARB or sacubutril valsartan, with beta-blockers, and, if tolerated, MRAs
Originally a treatment for diabetes, benefits of reduced hospital admissions and mortality have been demonstrated in patients with HF-REF with no diabetes.
- Thought to be a class effect, however the evidence in this group only exists for dapagliflozin.
- Patients with diabetes already stable on another SGLT2 inhibitor need not switch to dapagliflozin to treat their HF-REF.
If 100 people like this take dapagliflozin for 18 months, 2.2 will avoid death compared with if they hadn't taken dapagliflozin
If 100 people like this take dapagliflozin for 18 months, 2.3 will avoid a cardiovascular death compared with if they hadn't taken dapagliflozin
If 100 people like this take dapagliflozin for 18 months, 3.7 will avoid hospitalisation for heart failure compared with if they hadn't taken dapagliflozin
If 100 people like this take dapagliflozin for 18 months, 4.8 will avoid cardiovascular death or hospitalisation for heart failure compared with if they hadn't taken dapagliflozin
Evidence Source: NICE
Figures derived from a single RCT (DAPA-HF)1 which informed a 2021 NICE technology appraisal2 (this treatment did not feature in the 2018 guideline).
- included 4,744 patients
- NHYA symptoms II, III and IV
- on other standard treatments (ACE+BB+MRA)
- 58% without diabetes
Benefits were comparable for those with and without diabetes (reaching statistical significance for both sub-groups).
References
1)McMurray JJV, Solomon SD, Inzucchi SE et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008
2)National Institute for Health and Care Excellence. Dapagliflozin for treating chronic heart failure with reduced ejection fraction 2021 [Internet]. [London]: NICE; 2021. (Technology appraisal guidance [TA679])
Evidence Quality: MODERATE
| This research provides a good indication of the treatment effect.
There is a moderate possibility that the true effect is smaller or greater. |
NICE rated this trial to be at low risk of bias and large enough to produce significant results. However,
- the results may not fully apply to all those with heart failure, as the trial population was younger and with with fewer comorbidities than may be seen in clinical practice.
Reference
1)National Institute for Health and Care Excellence. Dapagliflozin for treating chronic heart failure with reduced ejection fraction 2021 [Internet]. [London]: NICE; 2021. (Technology appraisal guidance [TA679])
Study Population
The population characteristics in this trial1 were:
- mean age 66
- 23% female
- ethnicity: White 70%, Black 5%, Asian 23%, other 2%
- NYHA II (67%), NHYA III (32%), NYHA IV (1%)
- mean LVEF 31% (sd 6.7)
- 56% IHD
- 42% diabetes
- 38% atrial fibrillation
- mean eGFR 66ml/min/1.73m2
- mean SBP 122mmHg
- 95% on ACEi or ARB or sacubutril valsartan
- 96% on beta-blocker
- 71% on MRA
Reference
1)McMurray JJV, Solomon SD, Inzucchi SE et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008
The DAPA-HF trial1 (which provides the data on benefits here), showed no increase in the following adverse events with dapagliflozin treatment:
- volume depletion
- renal adverse events
- hypoglycaemia (severe enough to need assistance to administer carbohydrates or glucagon)
- diabetic ketoacidosis
However, this was one trial in a particular population:
- See the sections on SGLT2 inhibitors in CKD and T2DM for further information on harms in these populations.
Reference
1)McMurray JJV, Solomon SD, Inzucchi SE et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008.
Specialist-initiated treatment
Sacubutril with valsartan (combined tablet) in place of ACEi or ARB.
NICE recommends this as an option for treating symptomatic HF-REF1, only in people:
- with NYHA symptom class II-IV and
- LVEF ≤35% and
- who are on stable treatment with an ACEi/ARB
There is evidence that in a carefully selected population it can reduce mortality and hospitalisations.
It is likely that at least 40% of patients will not be able to tolerate this medication at trial doses (see “Evidence Quality” below).
If 100 people like this take sacubutril with valsartan for 2 1/4 years, 2.8 will avoid death compared to those taking enalapril
If 100 people like this take sacubutril with valsartan for 2 1/4 years, 3.2 will avoid a cardiovascular death compared to those taking enalapril
If 100 people like this take sacubutril with valsartan for 2 1/4 years, 2.8 will avoid hospitalisations for heart failure compared to those taking enalapril
If 100 people like this take sacubutril with valsartan for 2 1/4 years, 4.7 will avoid CV death or hospitalisation for HF compared to those taking enalapril
Evidence Source: NICE
Figures derived from a single RCT (PARADIGM-HF)1 which informed a 2016 NICE technology appraisal2 (referenced in the 2018 guideline):
- 4,399 patients included
- sacubutril with valsartan (mean dose 375mg) compared to enalapril (mean dose 19mg)
- NHYA symptoms I-IV (but mainly II and III)
- on other standard treatments (ACE+BB+MRA) at start of trial
References
1)McMurray JJV, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004
2)National Institute for Health and Care Excellence. Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction 2016 [Internet]. [London]: NICE; 2016. (Technology appraisal guidance [TA388])
Evidence Quality: MODERATE
NICE rated this as HIGH quality with clinically and statistically significant results1.
- The evidence is HIGH quality if applied to a population like the trial population, who are able to tolerate the medication.
However, the trial population were highly selected:
- younger than average with fewer comorbidities than may be seen in clinical practice
- 20% of enrolled patients were excluded for not tolerating treatment during a run-in period
- a further 18% of patients discontinued treatment during the trial
- both treatment arms had roughly similar discontinuation rates
- higher doses of both drugs were used than is normal in clinical practice
Therefore, this evidence may be less applicable to a general clinical population, meaning:
- there is a high possibility that the true effect is smaller or greater
- there is a higher possibility of side effects or medicine discontinuation.
Reference
1)National Institute for Health and Care Excellence. Sacubutril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction 2016 [Internet]. [London]: NICE; 2016. (Technology appraisal guidance [TA388])
Study Population
The population characteristics in this trial1 were:
- mean age 64
- 22% female
- ethnicity: White 66%, Black 5%, Asian 18%, other 11%
- NYHA I (4%) NYHA II (72%), NHYA III (23%), NYHA IV (1%)
- mean LVEF 29% (sd 7)
- 60% IHD
- 35% diabetes
- 37% atrial fibrillation
- mean creatinine 100umol/L
- mean SBP 122 mmHg
- all on ACEi or ARB, switched to enalapril or sacubutril+valsartan in trial
- 93% on beta-blocker
- 56% on MRA
Reference
1)McMurray JJV, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004
In the PARADIGM-HF trial1, sacubutril with valsartan was associated with:
- more hypotension than enalapril
- slightly less renal impairment and hyperkalaemia
- but side effect rates are likely to be higher in older, more complex patients as compared to trial populations
| Enalapril 19mg | Sacubutril with valsartan 375mg | Absolute risk difference with S+V | Number Needed to Harm |
|
| Symptomatic hypotension | 9.2% | 14% | +4.8% | 21 |
| Creatinine >221umol/L | 4.5% | 3.3% | -1.2% | 83* |
| K+ >6.0mmol/L | 5.6% | 4.3% | -1.3% | 77* |
Reference
1)McMurray JJV, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004
Specialist-initiated treatment
Ivabradine is a sinus-node inhibitor which slows heart rate. It has a specific recommendation in HF-REF:
- with NYHA symptom class II-IV and
- in sinus rhythm with rate ≥75bpm and
- LVEF ≤35% and
- who are on stable treatment with an ACEi/ARB, beta-blocker and MRA (or if beta-blocker not tolerated)
In this carefully selected population it can reduce hospitalisations for heart failure and mortality.
If 100 people like this take ivabradine for 2 1/4 years, 5 will avoid a hospital admission for heart failure compared with if they hadn't taken ivabadrine
If 100 people like this take ivabradine for 2 1/4 years, 1.1 will avoid a death from heart failure compared with if they hadn't taken ivabadrine
Evidence: NICE
Figures derived from a single RCT (SHIFT)1 which informed a 2010 NICE technology appraisal2 (referenced in the 2018 guideline3):
- 6558 patients included
- compared ivabradine 6.5mg bd with placebo
- NHYA symptoms I-IV (mainly II and III)
- on other standard treatments (ACE+BB+MRA) at start of trial
References
1)Swedberg K, Komajda M, Böhm M et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744): 875-885
2)National Institute for Health and Care Excellence. Ivabradine for treating chronic heart failure 2010[Internet]. [London]: NICE; 2010. (Technology appraisal guidance [TA267])
3)National Institute for Health and Care Excellence. Chronic heart failure in adults: diagnosis and management 2018 [Internet]. [London]: NICE; 2018. (NICE guideline [NG106])
Evidence Quality: HIGH
NICE rated this as HIGH quality and to have produced clinically and statistically significant results1, however it is worth noting that:
- the trial population was younger than average with fewer comorbidities than may be seen in clinical practice
- mortality reduction was only statistically significant in a post-hoc subgroup analysis of those with pulse rate ≥75bpm (data not available)
Reference
1)National Institute for Health and Care Excellence. Ivabradine for treating chronic heart failure 2010[Internet]. [London]: NICE; 2010. (Technology appraisal guidance [TA267])
Study Population
The population characteristics in this trial1 were:
- mean age 60
- 24% female
- ethnicity: White 89%, Asian 8%, other 3%
- NYHA II (49%), NHYA III (50%), NYHA IV (1%)
- mean LVEF 29% (sd 5)
- 67% IHD
- 30% diabetes
- 8% atrial fibrillation
- mean eGFR 75 ml/min/1.73m2
- mean SBP 122 mmHg
- 92% on ACEi or ARB
- 90% on beta-blocker
- 60% on MRA
Reference
1)Swedberg K, Komajda M, Böhm M et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744): 875-885
Side effects reported in the SHIFT trial1 :
| Placebo | Ivabradine | Absolute Risk Increase | Number Needed to Harm |
|
| Symptomatic bradycardia | 1% | 4.6% | 3.6% | 27 |
| Atrial fibrillation | 7.7% | 9.4% | 1.8% | 57 |
| Phosphenes* | 0.5% | 2.7% | 2.2% | 45 |
*transient enhanced brightness in a restricted area of the visual field
- MODERATE quality evidence
Reference
1)Swedberg K, Komajda M, Böhm M et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744): 875-885
Key
People who have an adverse event
People whose adverse event is prevented by treatment
People who were never going to have an adverse event anyway
Graphics and NNTs are rounded to the nearest integer
Stats explained
These three statistical terms offer three different ways of looking at the results of trial data.
ARR
Absolute Risk Reduction
This tells you how many people out of 100 who take a treatment have an adverse event prevented.
MoreThe value of the ARR changes with the baseline risk of the person (or population) taking the treatment. The higher the starting risk, the greater the absolute chance of benefit.
You need to think about over what time the trial data show this benefit, as it is usually assumed that more absolute risk reduction is gained over time.
Your patient might be taking the treatment for much longer than the length of a clinical trial (or, if life expectancy is limited, perhaps for less time).
NNT
Number Needed to Treat
This tells you how many people need to take the treatment in order for one person to avoid an adverse event.
The lower the number, the more effective the treatment.
MoreThe value of the NNT changes with the baseline risk of the person (or population) taking the treatment. The higher the starting risk, the smaller the NNT.
You need to think about over what time the trial data show benefit, as it is usually assumed that more benefit is gained over time and therefore the NNT will drop over time.
Your patient might be taking the treatment for much longer than the length of a clinical trial (or, if life expectancy is limited, perhaps for less time).
RRR
Relative Risk Reduction
This tells you the proportion of adverse events that are avoided if the entire population at risk is treated.
MoreThe value of the RRR is usually constant in people (or populations) at varying degrees of risk.
It is also usually assumed to stay constant over time.
This can be helpful, especially when thinking about population outcomes, but can be misleading for an individual person:
For example, a RRR of 25% in someone with a baseline risk of 40% would give them an ARR of 10% and an NNT of 10.
A RRR of 25% in someone with a baseline risk of 4% would give them an ARR or 1% and an NNT of 100.